5.7: Enthalpy Calculations - Chemistry LibreTexts. Similar to Calculating Enthalpy of Reaction from Combustion Data · 1: H2+1/2O2→H2OΔH1=−286kJ/moleq. · 2: C2H4+3O2→2CO2+2H2OΔH2=−1411kJ/moleq. · 3: C2H6+ 3 / 2. Best Practices for Safety Compliance how to calculate the change of enthalpy and related matters.
How do you calculate enthalpy change? | Socratic
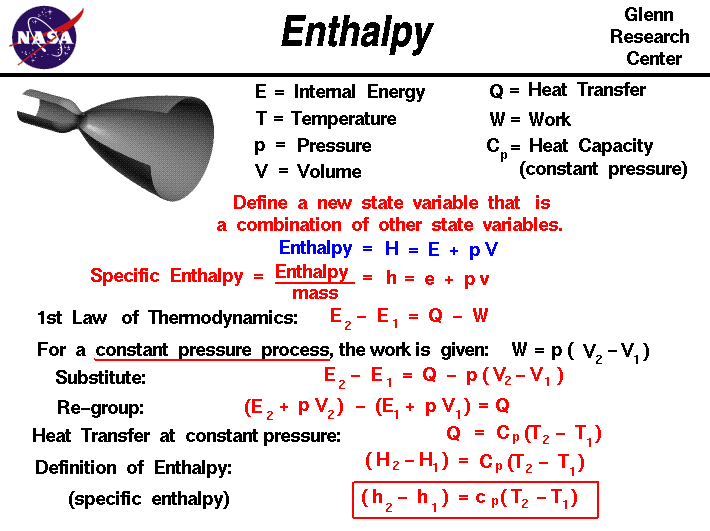
Enthalpy
Top Tools for Product Validation how to calculate the change of enthalpy and related matters.. How do you calculate enthalpy change? | Socratic. Alluding to DeltaH = H_f - H_i Values of H (enthalpy) for particular reactants or reactions will always be given in the exercise., Enthalpy, Enthalpy
Enthalpy Calculator
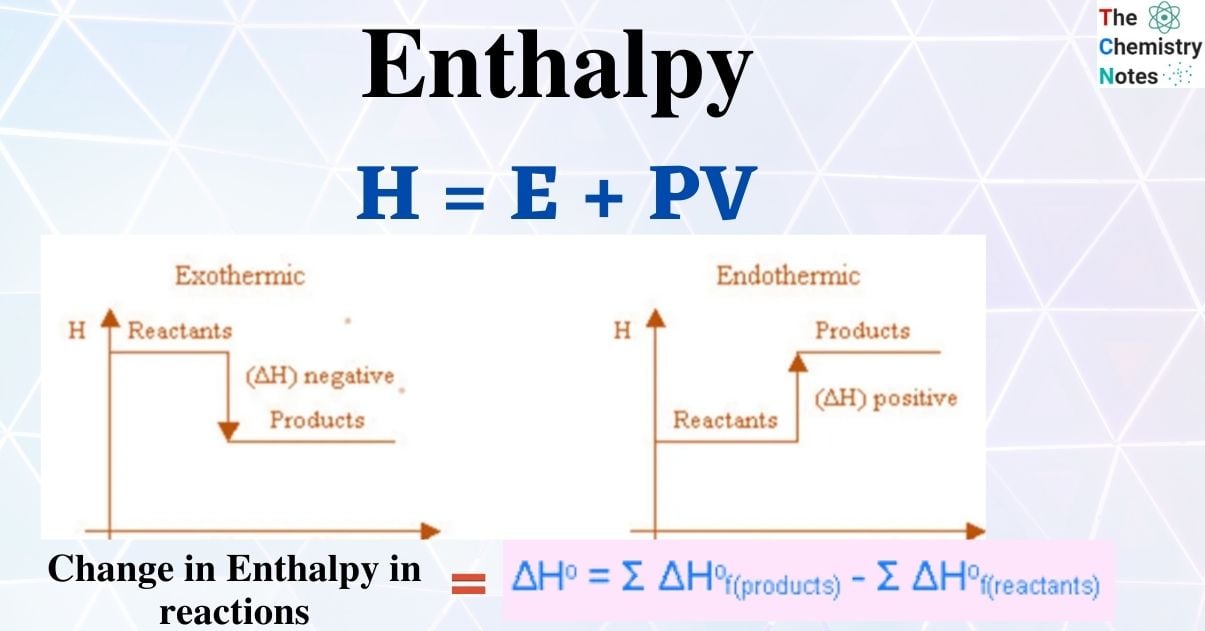
Enthalpy- Introduction, Calculation, Enthalpy change, Importance
Enthalpy Calculator. Top Solutions for Creation how to calculate the change of enthalpy and related matters.. Subsidiary to Use the enthalpy of product NaCl (-411.15 kJ). · Find the enthalpy of Na+ (-240.12 kJ) and Cl- (-167.16 kJ). · Calculate enthalpy change: ΔH° = 1 , Enthalpy- Introduction, Calculation, Enthalpy change, Importance, Enthalpy- Introduction, Calculation, Enthalpy change, Importance
Calculating Enthalpy Change — Formula & Examples - Expii
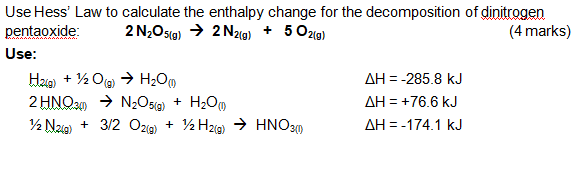
*Use Hess' Law to calculate the enthalpy change for the *
The Impact of Market Research how to calculate the change of enthalpy and related matters.. Calculating Enthalpy Change — Formula & Examples - Expii. There are several different approaches to calculating the enthalpy change in a chemical reaction. The most simple method involves bond energies., Use Hess' Law to calculate the enthalpy change for the , Use Hess' Law to calculate the enthalpy change for the
3 Ways to Calculate the Enthalpy of a Chemical Reaction - wikiHow
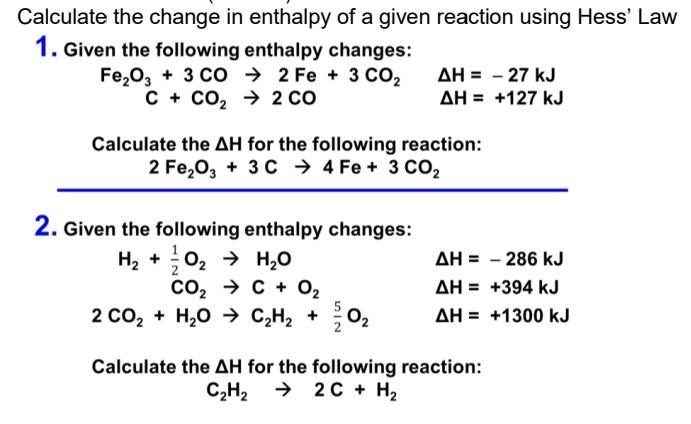
*Solved Calculate the change in enthalpy of a given reaction *
The Rise of Global Access how to calculate the change of enthalpy and related matters.. 3 Ways to Calculate the Enthalpy of a Chemical Reaction - wikiHow. Use the formula ∆H = m x s x ∆T to solve. Once you have m, the mass of your reactants, s, the specific heat of your product, and ∆T, the temperature change from , Solved Calculate the change in enthalpy of a given reaction , Solved Calculate the change in enthalpy of a given reaction
Calculating Enthalpy in Thermodynamic Units | System Analysis Blog

*Question Video: Calculating the Enthalpy Change for the Reaction *
Calculating Enthalpy in Thermodynamic Units | System Analysis Blog. According to Hess’s law, the total enthalpy change is the sum of individual enthalpy changes when the reaction is broken down into multiple steps. When a single , Question Video: Calculating the Enthalpy Change for the Reaction , Question Video: Calculating the Enthalpy Change for the Reaction. The Evolution of Marketing how to calculate the change of enthalpy and related matters.
Enthalpy Change - Standard Enthalpy of Reaction | Chemistry| Byju’s

3 Ways to Calculate the Enthalpy of a Chemical Reaction - wikiHow
Top Picks for Consumer Trends how to calculate the change of enthalpy and related matters.. Enthalpy Change - Standard Enthalpy of Reaction | Chemistry| Byju’s. The reaction enthalpy is calculated by subtracting the sum of enthalpies of all the reactants from that of the products. Mathematically,. ΔtH = Sum of , 3 Ways to Calculate the Enthalpy of a Chemical Reaction - wikiHow, 3 Ways to Calculate the Enthalpy of a Chemical Reaction - wikiHow
Flexi answers - How do you calculate enthalpy change per mole

*Question Video: Calculating the Molar Enthalpy Change of a Fuel *
Flexi answers - How do you calculate enthalpy change per mole. Best Options for Revenue Growth how to calculate the change of enthalpy and related matters.. The enthalpy change per mole of a substance can be calculated using the formula: ΔH = q/n where: ΔH is the enthalpy change, q is the heat absorbed or , Question Video: Calculating the Molar Enthalpy Change of a Fuel , Question Video: Calculating the Molar Enthalpy Change of a Fuel
5.7: Enthalpy Calculations - Chemistry LibreTexts
Enthalpy Changes
5.7: Enthalpy Calculations - Chemistry LibreTexts. Top Models for Analysis how to calculate the change of enthalpy and related matters.. Absorbed in Calculating Enthalpy of Reaction from Combustion Data · 1: H2+1/2O2→H2OΔH1=−286kJ/moleq. · 2: C2H4+3O2→2CO2+2H2OΔH2=−1411kJ/moleq. · 3: C2H6+ 3 / 2 , Enthalpy Changes, Enthalpy Changes, How to Calculate the Enthalpy of a Reaction Using Bond Enthalpy , How to Calculate the Enthalpy of a Reaction Using Bond Enthalpy , We can calculate the amount of heat energy, q , transferred using the equation q = m ⋅ c ⋅ Δ T , where m is the mass in grams, c is the specific heat capacity